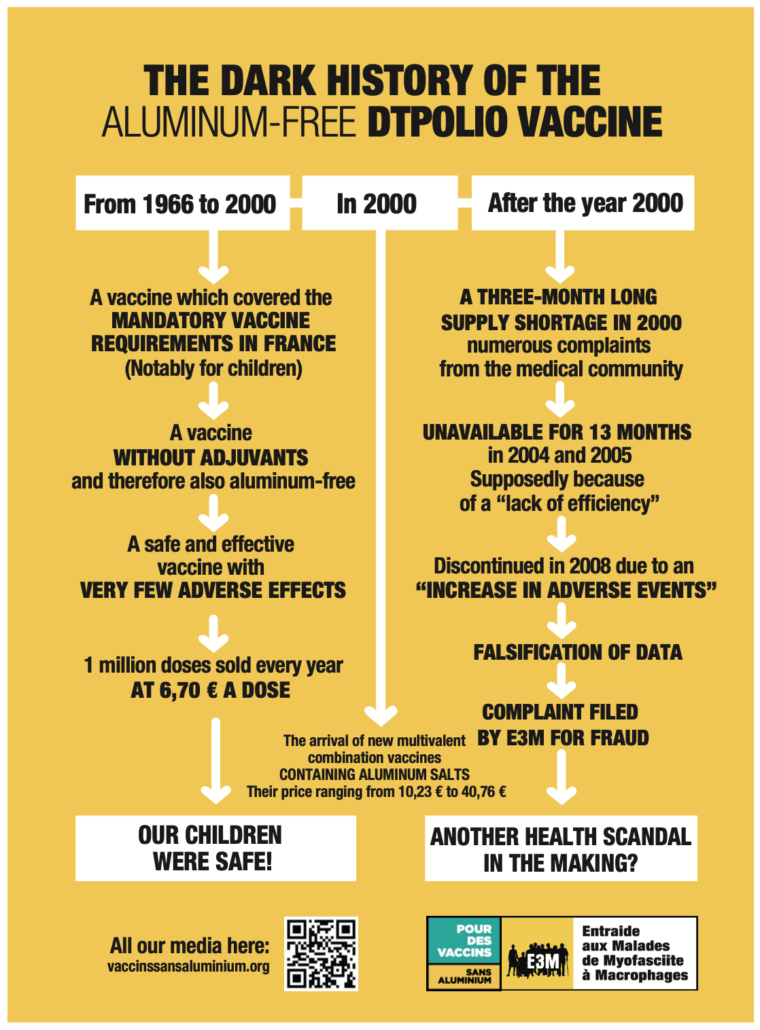
The aluminum-free DTPolio vaccine discontinued: falsified data
6 September 2015 by E3M
Background
Alerts about the aluminum adjuvants in vaccines are important enough to prompt population protection measures. Aluminum free vaccines must be made available, especially for children subject to vaccine requirements.
Marisol Touraine, a French politician who has previously served as Minister of Social Affairs and Health in France, made a commitment during the election campaign: families “must have the possibility to choose aluminum free vaccines when they fulfill the mandatory vaccine requirements, especially since this was a possibility up until 2008”.
The marketing of the aluminum free DTPolio vaccine was discontinued in France on 12 June 2008 by the ANSM (the French Medecines agency) at the request of Sanofi Pasteur MSD, following a supposedly “significant increase in adverse reactions”.
General information about adverse reactions
All vaccines, like any other drug, has adverse effects.
The adverse effects of the aluminum-free DTPolio vaccine are allergic in nature. These are the kind of adverse effects mentioned in all vaccine package leaflets.
The usual number of adverse reactions for the aluminum-free DTPolio vaccine is between 3 and 4 per 100,000 doses, depending on the year. For comparison, the numbers for Gardasil, the papillomavirus vaccine, is 39.9 per 100 000 doses (2013).
The DTPolio Mérieux vaccine, free from aluminum, taken off the market
The withdrawal of the aluminum free DTPolio vaccine was officially justified by a significant increase in adverse reactions in early 2008: it was said that the number of adverse reactions had tripled to 10.7 per 100,000 doses (note: without any increase in serious adverse events).
An analysis of official data provided by the French Medicines Agency (AFSSAPS/ANSM) to E3M shows that:
- 6 batches of DTPolio vaccines were sold in early 2008;
- These 6 batches were also marketed in 2007;
- The number of adverse events over the life span of these 6 lots is 3.78 per 100,000 doses, which is in the usual average for the DTPolio vaccine, which has been on the market for 47 years.
The same official data show a surprising distribution of adverse reactions:
- In 2008: 10.7 adverse reactions per 100,000 doses;
- In 2007: 1.08 adverse reactions per 100,000 doses;
- Whereas 28% of the doses were sold in 2008, and 72% in 2007.
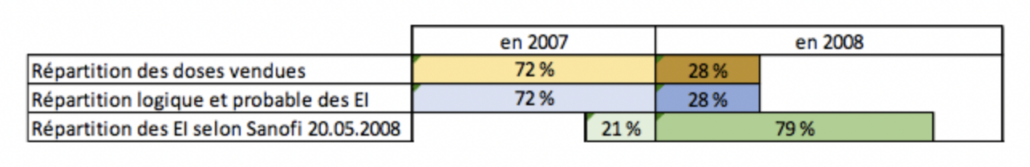
EI = Effets Indésirables = Adverse Effects
On a single batch of vaccines, it is conceivable that the adverse reactions would not be randomly distributed, for example following a cold chain interruption. But on 6 lots, the probability of such an irregular distribution is 1.16/100,000.
These elements led E3M to file a complaint against X for « faux, usage de faux, et escroquerie » (falsification and fraud) before the Public Prosecutor. In addition, E3M has joined with Karen BOUILLOT and Stanley ANNAN in a civil action for “Personal Injury and endangerment of others », because the new DTP vaccine (Revaxis) contains aluminum, and that the presence of aluminum in this vaccine has been proven to cause macrophage myofascitis (an inflammatory, often debilitating, syndrome characterized by muscle pain and extreme fatigue) in these two people.

The marketing authorizations for the 2 aluminum-free DTP vaccines are still valid
The Drug Agency (ANSM) and the Directorate-General for Health (DGS) claim that the marketing authorization (MA) of DTPolio Mérieux vaccines (and DTP Pasteur, which was a copy of the DTPolio Mérieux vaccine) are now obsolete, because Sanofi Pasteur MSD has not requested the renewal of these marketing authorizations at the scheduled dates (12/06/2011 for DTPolio Mérieux, 29/11/2012 for DTP Pasteur).
We contest this official position on the basis of article R 5121-45 of the Public Health Code: « Once renewed, the marketing authorization is granted without limitation in time. However, the Agency may, on renewal, decide, in particular for reasons relating to pharmacovigilance, for example if an insufficient number of patients are exposed to the medicinal product or product concerned, that this authorization must be renewed every five years.”
When reading the marketing authorization renewal notifications, they do not contain a five-year renewal request. Therefore, they are still valid.
The probable motives for this falsification of data
According to our analysis, two reasons may be at the origin of the discontinuation of the aluminum-free DTPolio vaccine:
- A desire to rationalize production, through the disappearance of a majority of monovalent vaccines or vaccines that combine only a few virus strains (« small » combination vaccines or “atypical” vaccines);
- An immediate financial interest, by shifting users to more expensive vaccines.
But the aluminum-free DTPolio vaccine met a mandatory vaccine requirement as well as a demand from the medical profession. For decades it sold up to 1 million doses annually.
It took Sanofi Pasteur 8 years to achieve its goals:
- Supply shortage in the year 2000. A failed attempt which was followed by negative reactions from doctors and government officials.
- “Procurement challenges” in 2004 for so-called “lack of efficiency”. Another failure since the aluminum-free DTPolio proves to be more effective than Revaxis in primary vaccination, and also effective when used as a booster.
- Taken off the market in 2008 for “increased adverse effects”. This is an argument which will naturally scare health authorities. However, it has been shown that the underlying data was falsified.
It is therefore essential that the aluminum-free DTPolio be put back on the market, for the sake of all children entering the public school system (mandatory vaccinations in order to attend school), but also for all citizens who do not wish to be vaccinated with aluminum-containing vaccines. This has been one of E3M’s battles since 2008. Furthermore, light must be shed on the falsification of important data.