Death post-Gardasil (2023): Is the doctor the only one to blame?
March 8, 2025/ by E3M
On October 19, 2023, young E. (12 years old) receives an injection of Gardasil (human papillomavirus vaccine), in his secondary school in Saint Herblain (Loire-Atlantique, France). Shortly after the injection, he feels faint and falls down. Suffering from a traumatic brain injury, he passes away on 27 October at the Nantes hospital.
On March 6, 2025, Ouest France informs us that the doctor present at the vaccination session is being investigated for manslaughter. This doctor “may have failed in his duty and is said to have left the two nurses to fend for themselves in this moment of extreme gravity”.
The risk is great that this doctor will become the scapegoat. But to us, it seems that this drama must be analyzed through a triple lens: the individual fault, the responsibility of the State and the responsibility of MSD, the manufacturer of Gardasil.
Individual fault
Justice will decide whether or not there is human error. This may be the case for the post-vaccination surveillance, as it appears to be here, but also for the risk analysis prior to any injection. The European Court of Justice recently reminded us that: « the doctors who administered these vaccines [against COVID in this specific case] without any prior individual evaluation of the patient could be held liable. Indeed, the fact that these vaccines had received a marketing authorization (MA) was not sufficient to exempt practitioners from their duty to conduct a risk analysis for each patient” (Judgment of the ECJ of 30 January 2025). A decision that will set precedent regardless of the type of vaccine involved.
The responsibility of the state
E3M opposes once again the practice of mass vaccinations, even more so in a place intended for education, preceded by extensive publicity campaigns and strong media coverage from the State. Any vaccination should be performed by the treating physician (or a para-medical professional, after prescription by the treating physician). It is a guarantee of safety, on the one hand because the attending physician knows his patient (and therefore any contraindications that are specific to him), and on the other hand he is used to administering vaccines and knows how to behave in the case of an allergic reaction.
The responsibility of MSD, the manufacturer of Gardasil
We were very surprised by the statement of the Regional Health Agency, shortly after the accident, that this death was “unrelated to the vaccine product or a defect in the quality of the vaccine” (France Bleu). What analysis had been done to make this statement? This looks a bit too much like the Pavlovian reflex we know so well: “vaccines are safe, vaccination is not up for discussion”. Well, actually it should be discussed, and especially in relation to the Gardasil and its many serious adverse effects (read our HPV file, and watch the video « Did you read the Gardasil vaccine insert? « ).
In an email sent to the Prosecutor shortly after the death was announced, E3M expressed concern: “the Food and Drug Administration vaccine insert mentions (1) syncope as an adverse effect of Gardasil – without any indication of a possible psychosomatic origin (stress, etc.), and (2) deaths following this vaccination.”
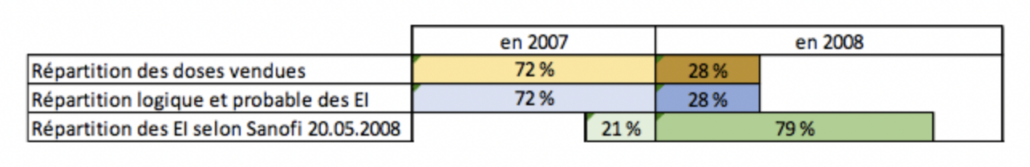
In a report made public, this concern is now amplified by the questioning of the quality of the safety studies carried out by the manufacturer (MSD) at the request of the European Medicines Agency and those carried out subsequently by the FDA and the CDC (North American agencies) using the same methodology. According to this document, Merck has made a biased selection of vaccine adverse reaction data to prove that its Gardasil HPV vaccine does not cause serious neurological effects. The FDA and the CDC used this same method of data selection for their own study, reaching the same conclusion (Source).
Review of the judicial consequences of E.’s death in Nantes
The prosecutor announced in 2023: «investigations will be conducted as to the origin of the malaise, which led to this fatal fall» (France Bleu). Aurélien Rousseau, then minister of health, promised: «everything will be examined and made public (Ouest France). As far as we know, the results of this investigation have not yet been made public.
We are asking for full transparency on this drama, in all its dimensions. The justice system should not limit itself to targeting one single doctor. We are calling for transparency even more so since the vaccination campaign in secondary schools is still on-going and could eventually even be expanded to include meningococcal vaccination (JIM – October 29, 2024).